Provider Alert! Clinical Prior Authorization Updates for Cystic Fibrosis Agents
Date: February 25, 2021
Attention: Pulmonologists
Effective Date: April 6, 2021
Providers should monitor the Texas Children’s Health Plan (TCHP) Provider Portal regularly for alerts and updates associated to the COVID-19 event. TCHP reserves the right to update and/or change this information without prior notice due to the evolving nature of the COVID-19 event.
Call to action: Effective April 6, 2021, the Texas Health and Human Services Commission (HHSC) will update the cystic fibrosis (CF) agents prior authorization criteria to reflect recent FDA-approved indication expansions. This impacts the following agents: Kalydeco (ivacaftor), Orkambi (lumacaftor and ivacaftor), Symdeko (tezacaftor and ivacaftor), and Trikafta (elexacaftor, tezacaftor, and ivacaftor).
| Drug | Change effective April 6, 2021 |
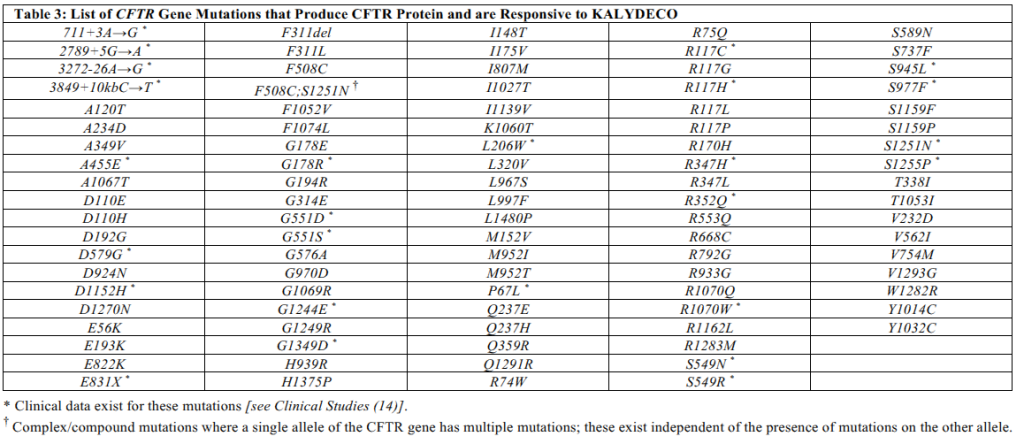
| Kalydeco (ivacaftor) | Cystic fibrosis (CF) patient age 4 months and older who has one mutation in the CFTR gene that is responsive to ivacaftor based on clinical and/or in vitro assay data. |
| Orkambi (lumacaftor and ivacaftor) | Cystic fibrosis (CF) patient age 2 years and older who is homozygous for the F508del mutation in the CFTR gene. |
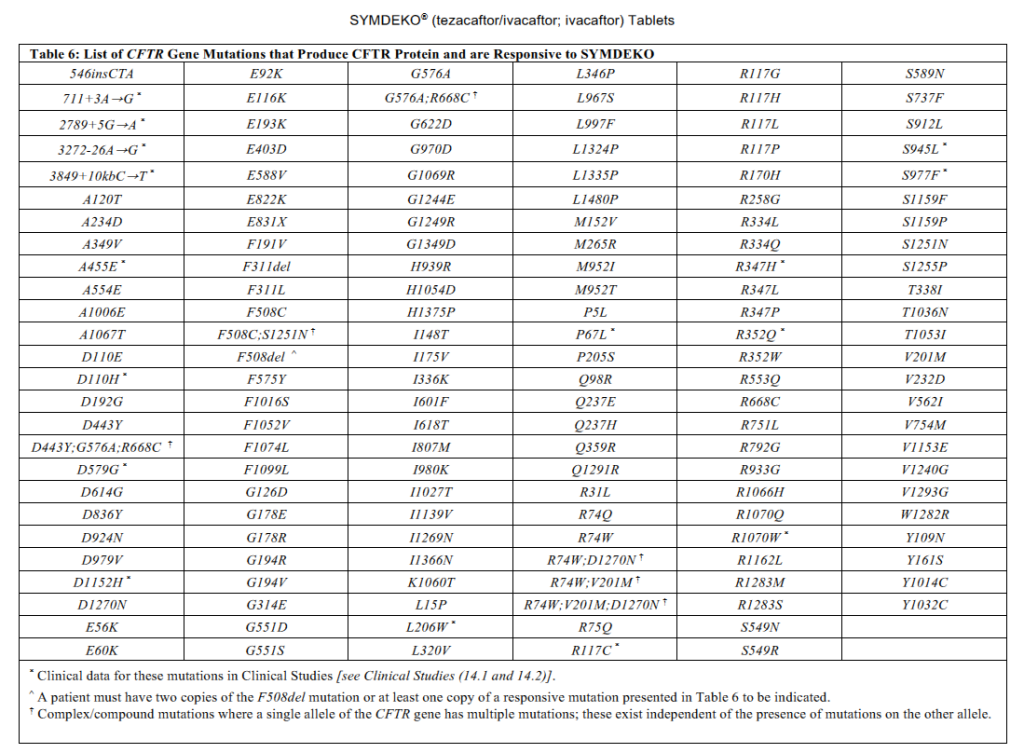
| Symdeko (tezacaftor and ivacaftor) | Cystic fibrosis (CF) patient age 6 years and older who is homozygous for the F508del mutation or who have at least one mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene that is responsive to tezacaftor/ivacaftor based on in vitro data and/or clinical evidence. |
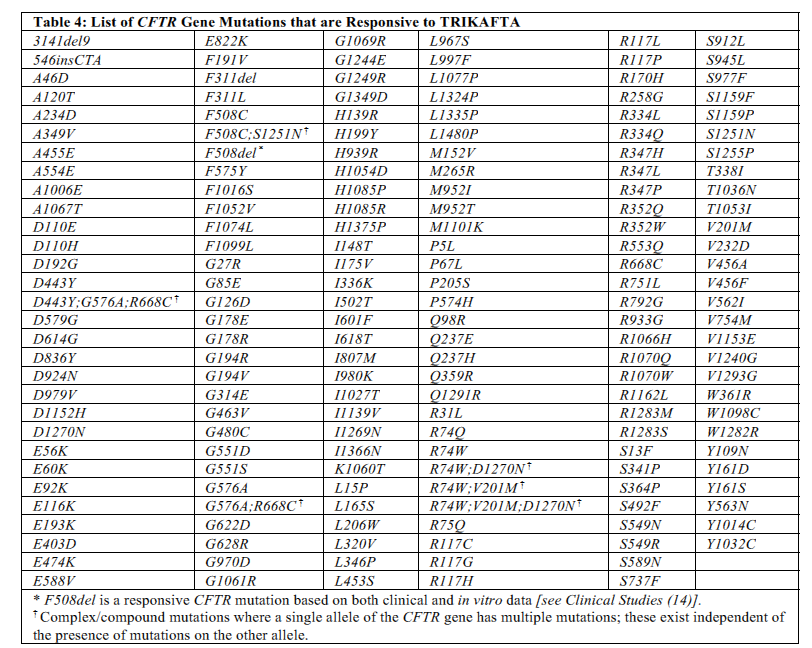
| Trikafta (elexacaftor, tezacaftor, ivacaftor) | Cystic fibrosis (CF) patients age 12 years and older who have at least one F508del mutation in the CFTR gene or a mutation in the CFTR gene that is responsive based on in vitro data. |
These agents will not be approved if the CFTR Genotype is unknown. An FDA-cleared CFTR gene mutation test should be performed to determine if a medication responsive disease causing CFTR gene mutation is present. Patients may be on only ONE of these agents at any one time.
Next steps for providers: Providers should check with TCHP for any changes to processing Medicaid claims. Providers should share this communication with their staff. The updated prior authorization forms will be available on the Navitus website.
Resources:
- https://txstarchip.navitus.com/pages/prior-authorization-forms.aspx
- List of CFTR Gene Mutations that produce CFTR protein responsive to various agents below. Information is from each medication’s package insert.
If you have any questions, please email TCHP Pharmacy Department at: tchppharmacy@texaschildrens.org.
For access to all provider alerts, log into:
www.thecheckup.org or www.texaschildrenshealthplan.org/for-providers.





Leave a Reply
You must be logged in to post a comment.